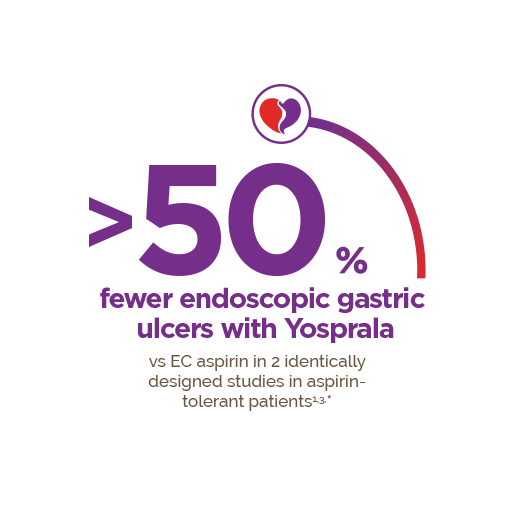
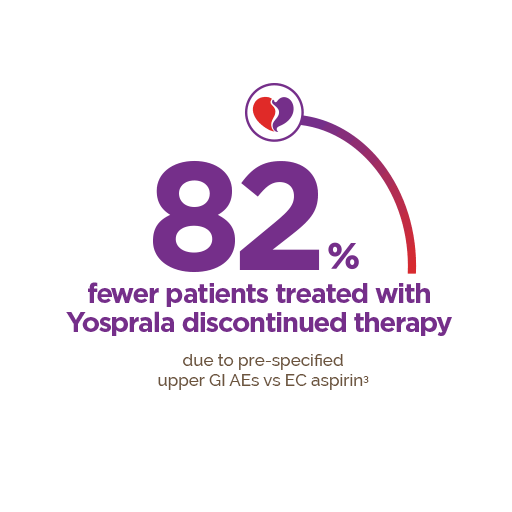
Reintroducing Yosprala® (aspirin and omeprazole), the ASA therapy for secondary prevention of cardiovascular events for patients at risk of developing aspirin associated gastric ulcers.1
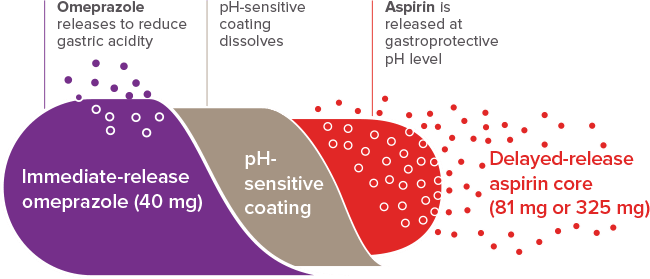
SEQUENTIAL DELIVERY FOR ADDED PROTECTION
Yosprala has not been shown to reduce the risk of gastrointestinal bleeding due to aspirin.1 ASA, Acetylsalicylic acid, CV, cardiovascular, GI, gastrointestinal.